Implant Augmentation -
Round Type
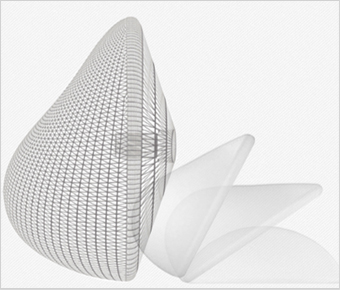
Characteristics of Round Implant
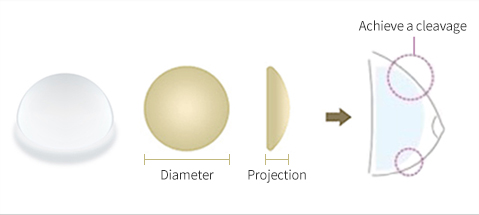
in order to achieve fullness of upper breasts with round bottom and curved
middle area. As the implant is perfectly round, the implant will emphasize the
natural shape, whether standing up or lying down.
It is appropriate for those who seek augmented breasts with full volume on the upper parts.